Are colloids heterogeneous. mixtures 2019-12-21
Colloid Examples in Chemistry

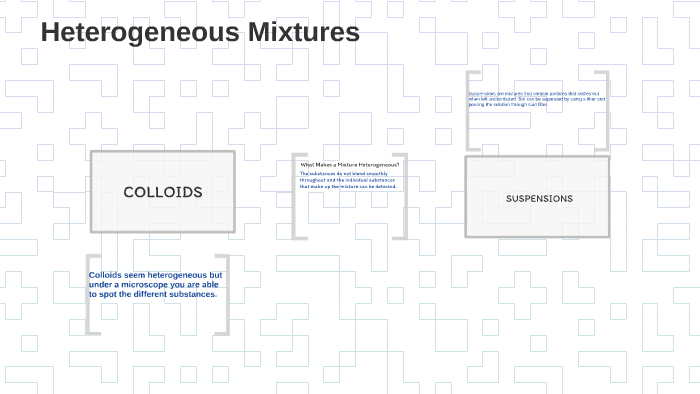
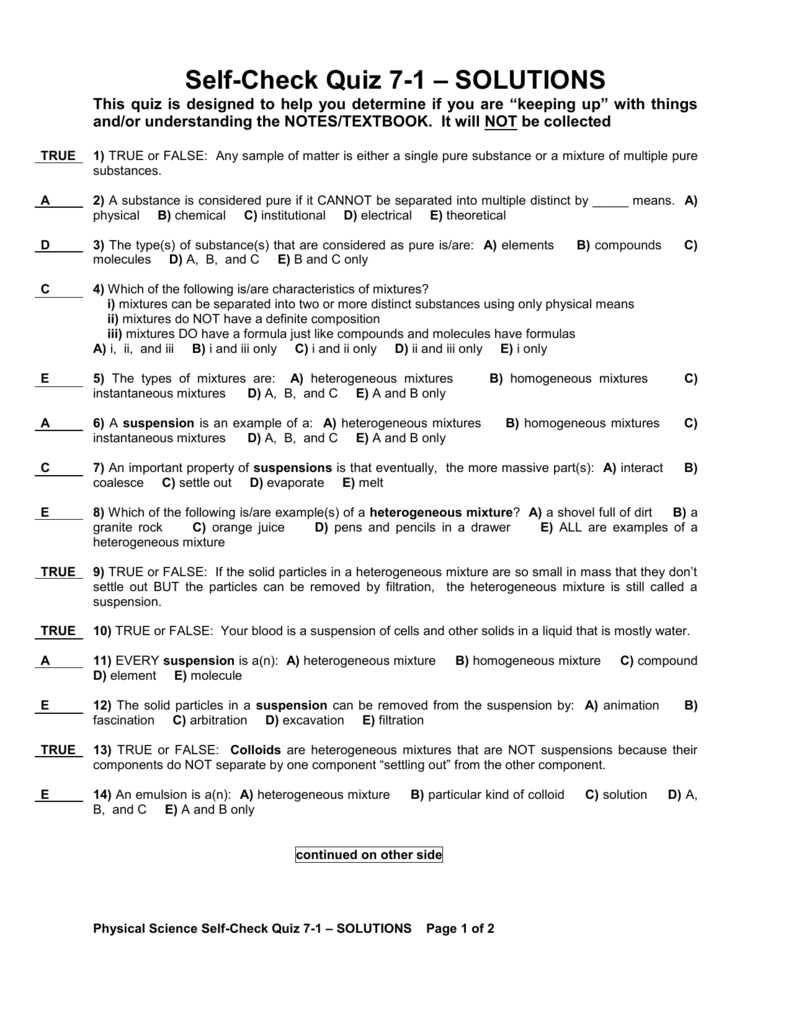
Provide details and share your research! I know a homogeneous mixture is a mixture in which only one visible phase is present, but why are suspensions and colloids considered heterogeneous, for you only see one phase as the definition states? Other substances which diffuse through parchment paper very slowly are called colloids. This is why, colloidal system appears as homogeneous mixtures, but in reality are heterogeneous mixtures. According to him, the substances which diffuse readily through a parchment membrane are called Crystalloids. Colloid is a heterogeneous mixture in which particle size is intermediate of true solution and suspension. Graham, while studying diffusion classified substances into two types: Crystalloids and Colloids. . An example of the Tyndall effect is the visibility of light from car headlamps through fog.
Next
Colloid Examples in Chemistry

There are two parts to every colloid mixture: the particles and the dispersing medium. Colloidal System Colloidal system is made up of Dispersed phase and Dispersion medium. In above example, particles of smoke are dispersed in air. Some common examples of colloids are gem stones, smoke, cheese, milk, soap lather and foam. To learn more, see our. Example: Soap shows colloidal character in water but crystalloid character in alcohol.
Next
mixtures

Thus size of colloids lies between that of true solution and suspension. Use MathJax to format equations. Is there a more accurate definition of a homogeneous and heterogeneous mixtures? Solutions and colloids don't separate. But this distinction was not valid, as under different conditions of temperature and pressure the same substance may act as crystalloid or colloid. The colloid particles are solids or liquids that are suspended in the medium.
Next
Colloid Examples in Chemistry

These particles are larger than molecules, distinguishing a colloid from a. For a polymer solution, you have to stretch this definition quite a bit already. Smoke from a fire is example of colloidal system in which tiny particles of solid float in air. For a colloidal solution even more. While colloidal mixtures are generally considered to be , they often display heterogeneous quality when viewed on the microscopic scale.
Next
mixtures
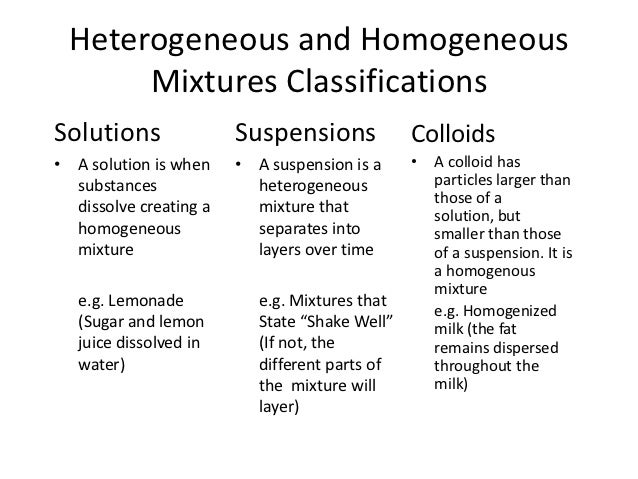
However, the particles in a colloid are smaller than those found in a. It has been realized that absolute distinction between Colloids and Crystalloids does not exist. Gelatin and glue are examples of Colloid. In colloidal system one substance is dispersed as very fine particles in another substance called dispersion medium. Brief History of Colloids In 1861, T.
Next
Colloid Examples in Chemistry

Example salts, sugars, acids and bases. I also know that homogeneous mixtures are usually referred to as solutions, but if only solutions are homogeneous then why do we define homogeneous as anything with 1 phase; isn't this misleading? In smoke, for examples, solid particles from combustion are suspended in a gas. I understand that the definition of a heterogeneous mixture is a mixture in which 2 or more phases are present, i. . . .
Next
What is a Colloid?

. . . . . . .
Next
Colloid Examples in Chemistry
. . . . .
Next
Colloid Examples in Chemistry
. . . . . .
Next
Colloid Examples in Chemistry

. . . . . .
Next









