What did niels bohr contribute to the atomic theory. Niels Bohr and the Manhattan Project 2019-12-20
Niels Bohr: Biography & Atomic Theory

Bohr died in Copenhagen on 18 November 1962, aged 77, and is buried in the Assistens Cemetery in Copenhagen. This quantum leaping in and out of existence can be very unnerving. The two Bohr brothers were always very close and both became influential professors at the University of Copenhagen; Neils in the field of physics and Harald in mathematics. If atoms don't already have eight atoms in their outer electron shell, they tend to bond with other atoms to complete the outer shell and be more stable. He studied the subject throughout his undergraduate and graduate years and earned a doctorate in physics in 1911 from Copenhagen University. Having studied the ideas presented by Planck and Einstein, Bohr wanted to theorize about the quantum property of all forms of energy.
Next
What did Bohr Contribute to the Theory of an Atom

It forces us to change our thinking in order to find it. The two physicist also grappled at this time with the philosophical implications of , and the extent to which it reflected the reality of the everyday world. Early Work By 1911, Bohr completed his doctorate at University of Copenhagen and became a post-doctoral student at Cambridge. He also played a large role in the development of atomic weapons and nuclear energy, and, in 1957, was awarded the Atoms for Peace Award for his efforts in the responsible use of atomic energy. Thompson in England when he was introduced to Ernest Rutherford, whose discovery of the nucleus and development of an atomic model had earned him a Nobel Prize in chemistry in 1908. But Bohr realized, like Planck and Einstein, that this energy can only come in chunks or quanta. He believed that nations should be completely open with one another and wrote down these views in his Open Letter to the United Nations in 1950.
Next
Bohr

In the same year that he began his studies with Rutherford, Bohr married the love of his life, Margaret Nørlund, with whom he had six sons. It has since been renamed the in his honor. According to his liquid droplet theory, a liquid drop provides an accurate representation of an atom's nucleus. Mindwarps The quantum nature of the universe is not limited to the subatomic world. Combining Rutherford's description of the nucleus and Planck's theory about quanta, Bohr explained what happens inside an atom and developed a picture of atomic structure.
Next
The Contributions of Neils Bohr to Chemistry
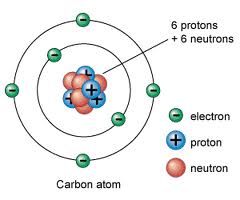
Churchill in particular was vehemently against sharing such secrets with Soviet Russia and considered Bohr as potentially unstable and a dangerous security risk. The current one that had been developed by Ernst Rutherford needed some tweaking to explain how atoms could emit light and yet not collapse in on themselves. Whenever possible, I linked to books with my amazon affiliate code, and as an Amazon Associate I earn from qualifying purchases. In October of 1885 in Copenhagen, Denmark, Christian and Ellen Bohr welcomed a new baby boy to their family and named him Niels. He was the first to discover that electrons travel in specific orbits. Cosmonotes We've used our imagination and have done some thought experiments to understand some of the theories we've discussed so far. He improved upon these ideas and sent them to the Royal Society in London, who published them in the journal Philosophical Transactions of the Royal Society in 1908, according to.
Next
The Contributions of Neils Bohr to Chemistry

He helped clarify several problems with quantum physics, including complementarity and fission. Examples of these types of atoms are the noble gases, like neon and argon. The Institute soon became an international focal point for theoretical physicists in the 1920s and 1930s, and most of the world's best known theoretical physicists of that period spent at least some time there. Raised in a family of academics, Bohr benefited from asking questions and learned about the importance of experimentation. The electron, proton and the atomic nucleus were known. For his experiment, Bohr won an impressive prize and decided to puruse a career in physics and not philosophy. The Danish physicist made important contributions to the understanding of the structure and energy of the atom, for which he was awarded the Nobel Prize.
Next
What new information did Niels Bohr contribute to the understanding of the atom?
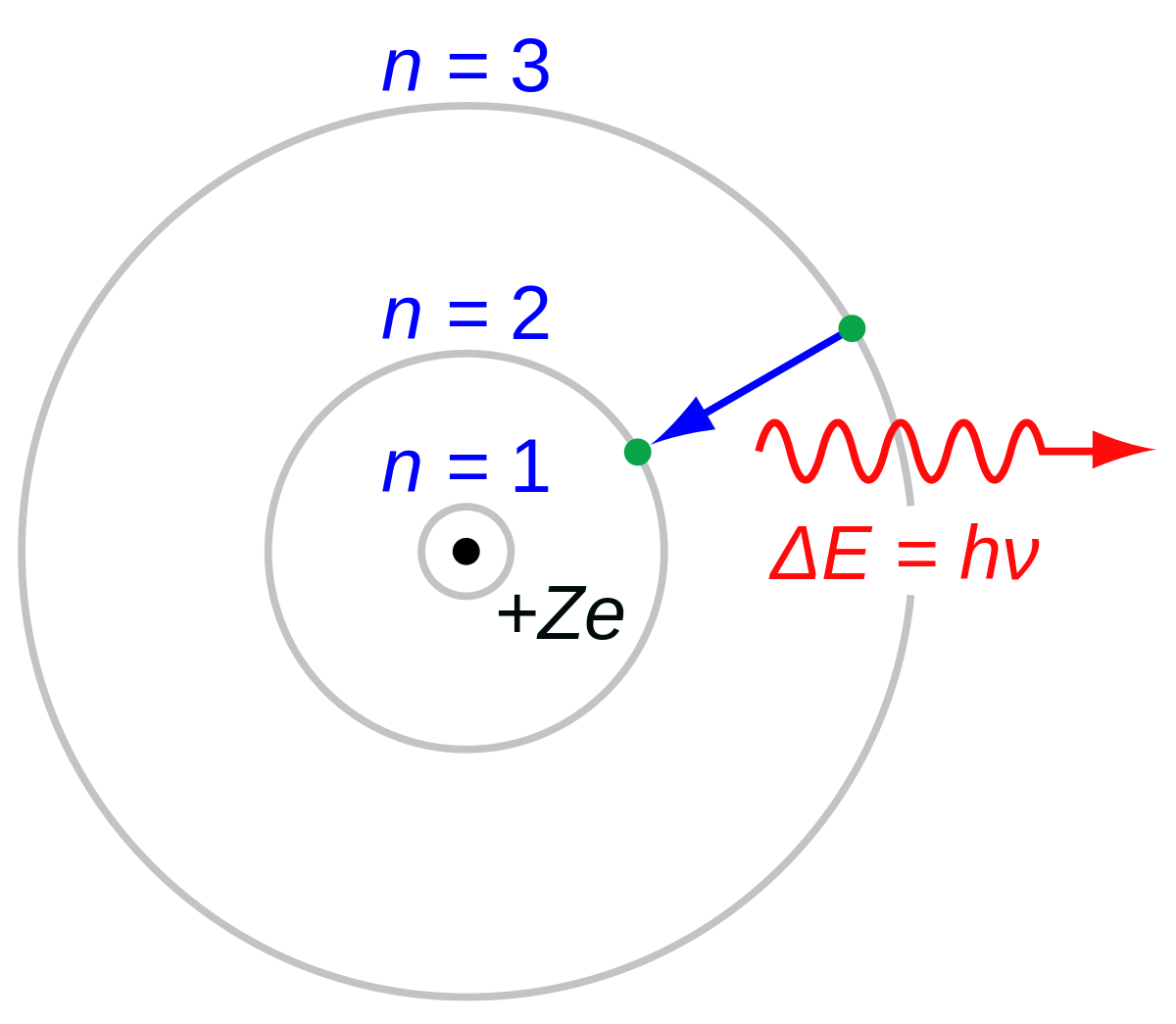
In 1912, Niels Bohr put forward a model to explain the structure of an atom. Bohr served as the director of the Institute for Theoretical Physics at the University of Copenhagen for many years, and after his death, it was renamed the Niels Bohr Institute. A jump from step four to step two gives off two steps' worth of energy or a jump from step five to step two gives off three steps of energy. While both deal with the nature of matter and energy, chemistry deals more with the interactions of atoms and their reactions in chemical processes. Later, Bohr would further his studies under the supervision of Ernest Rutherford, Nobel Prize winning chemist and discoverer of the proton. He went back to Copenhagen University in 1916 to become a professor of theoretical physics. This was different from prior ideas about the structure of atoms.
Next
What new information did Niels Bohr contribute to the understanding of the atom?

During the Second World War, Denmark was occupied by the German forces, and Bohr, who was quite aware of German nuclear research especially given his friendship with , who was intimately involved in German nuclear power research, although he was resisting involvement in the development of nuclear weapons , had to be very careful in his dealings and communications. She needs a minimum amount of energy before she can attain the next step or state. When jumping from one orbit to another with lower energy, a light quantum is emitted. While at Cambridge, Bohr first conducted experiments under the guidance of Sir Joseph John Thomson, Nobel Laureate and discoverer of the electron and of isotopes. The energy of an electron depends on the size of the orbit and is lower for smaller orbits. The chemical element bohrium Bh , No. This concept, which forms the basis of early quantum theory, also explains that regardless of how one views an electron, all understanding of its properties must be rooted in empirical measurement.
Next
Niels Bohr: Biography & Atomic Theory
For this pioneering work, Bohr earned the 1922 Nobel Prize in Physics. Virtually everything in the subatomic world is quantized. Despite his contributions to the U. Some electron shells require the electrons to have more energy than others, so if an electron absorbs some energy, it can move into a higher energy shell. Let's look at another analogy. When you're not really sure how something new operates, its often the best way to begin. In 1913, on the basis of 's theories, Bohr developed and published his model of atomic structure, known as the Bohr model, which depicts the as a small, surrounded by that travel in circular orbits around the , similar in structure to the Solar System, but with providing attraction, rather than.
Next
Bohr
:max_bytes(150000):strip_icc()/bohr-model-of-the-atom-603815_Final-5f95ec36a8874023b75caef27e337886.PNG)
While still a student, Bohr won a contest put on by the Academy of Sciences in Copenhagen for his investigation into the measurements of liquid surface tension using oscillating fluid jets. Neils Bohr is considered the grandfather of quantum mechanics. And some of them have traveled thousands of light years to get to you. Even when you look at a faint star, your eye receives a few hundred photons from that star each second. The first electron shell can contain two electrons, while the 2nd and 3rd can contain eight electrons. To this end, he had high level discussions with both the U. James Chadwick played a vital role in the atomic theory, as he discovered the Neutron in atoms.
Next
Niels Bohr
Liquid droplet theory Bohr's theoretical work contributed significantly to scientists' understanding of. Thomson the discoverer of the , and then at the University of Manchester under the discoverer of the and the structure of. Niels Bohr, with his wife, Margrethe After completing his Ph. An atom has to absorb energy by swallowing it whole and spits it back out in quantum chunks. If Planck is the father of quantum mechanics, then Bohr must be older than him, which in fact he's not, they're not even related-another strange quantum anomaly. This unanswerable characteristic is true of the entire microcosmic quantum world.
Next






