H2so4 ra h2s. Chemical Equation Balancer H2SO4 + HI = H2S + I2 + H2O 2019-12-16
Khí H2S là gì? Tác dụng và cách đo khí H2S!
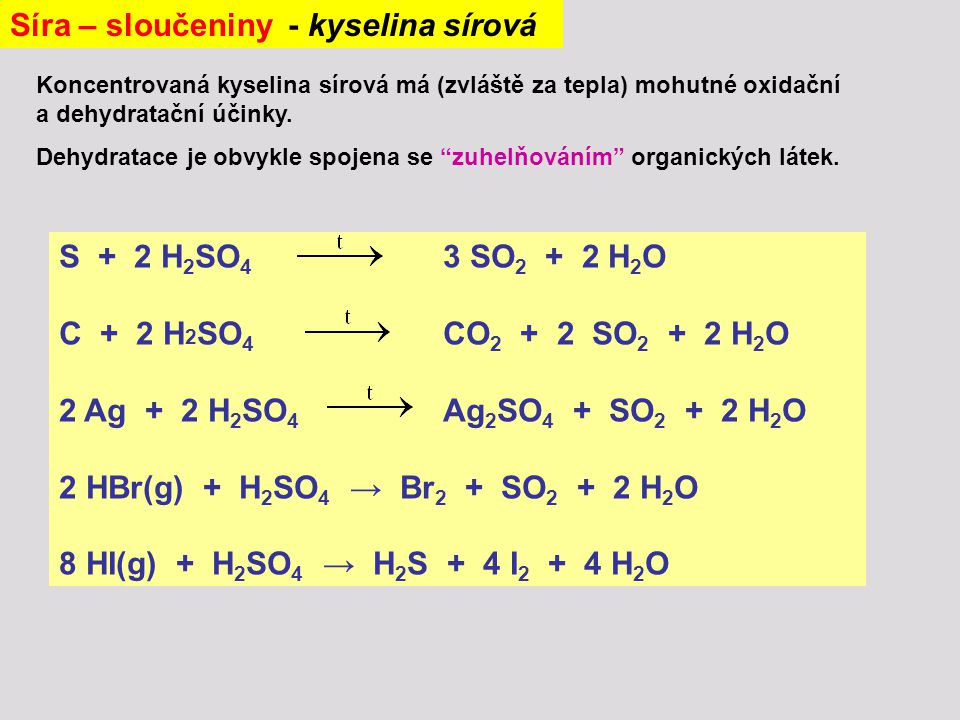
In common with other corrosive and , it readily decomposes and through and upon contact with , such as and. In 1746 in Birmingham, adapted this method to produce sulfuric acid in -lined chambers, which were stronger, less expensive, and could be made larger than the previously used glass containers. The reaction forms a corrosive aerosol that is very difficult to separate, instead of a liquid. Moreover, its makes it highly corrosive to many and may extend its destruction on other materials. In 1831, British merchant Peregrine Phillips patented the , which was a far more economical process for producing sulfur trioxide and concentrated sulfuric acid. The main cloud layer extends from 45—70 km above the planet's surface, with thinner hazes extending as low as 30 km and as high as 90 km above the surface. H2S cháy trong không khí với ngọn lửa màu xanh.
Next
Khí H2S là gì? Tác dụng và cách đo khí H2S!

This method does not produce an inseparable mist, which is quite convenient. H2S khan không tác dụng với Cu, Ag, Hg, nhưng khi có mặt hơi nước thì lại tác dụng khá nhanh làm cho bề mặt các kim loại này bị xám lại. A passage from ´s Summa Perfectionis was long considered to be a recipe for sulfuric acid, but this was a misinterpretation. The effect of this can be seen when concentrated sulfuric acid is spilled on paper which is composed of ; the cellulose reacts to give a appearance, the appears much as soot would in a fire. When it reacts with sulfur dioxide, a trace component of the Venusian atmosphere, the result is , which can combine with water vapor, another trace component of Venus's atmosphere, to yield sulfuric acid. The sugar changes from white to dark brown and then to black as carbon is formed.
Next
H2S + CuSO4 = CuS + H2SO4
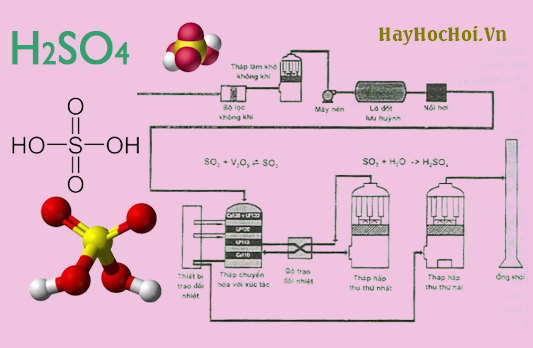
Because the reaction is in an equilibrium that favors the rapid protonation of water, addition of acid to the water ensures that the acid is the limiting reagent. Later refinements to the lead chamber process by French chemist and British chemist John Glover improved concentration to 78%. It is most commonly used in manufacture, but is also important in , , , and. Sulfuric acid is a very important commodity chemical, and a nation's sulfuric acid production is a good indicator of its industrial strength. The spinal cord is most often affected in such cases, but the optic nerves may show , loss of and.
Next
Chemical Equation Balancer H2SO4 + HI = H2S + I2 + H2O

In contrast, addition of water to concentrated sulfuric acid results in a thin layer of water on top of the acid. In 1736, , a London pharmacist, used this method to begin the first large-scale production of sulfuric acid. Sulfur trioxide is highly reactive and dissociates into sulfur dioxide and atomic oxygen, which oxidizes traces of carbon monoxide to form carbon dioxide. Concentrated sulfuric acid has a very powerful property, removing water from other including and other and producing , , and. It has a high , caused by dissociation through itself, a process known as. Pure sulfuric acid is a viscous clear liquid, like oil, and this explains the old name of the acid 'oil of vitriol'. This process is and must occur at high temperatures, so energy in the form of heat has to be supplied.
Next
H2S + CuSO4 = CuS + H2SO4

On-Line Learning Center for Organic Chemistry. Piranha solution is typically used in the microelectronics industry, and also in laboratory settings to clean glassware. Trường hợp nặng có thể gây ra tê liệt toàn thân và tử vong. Determination of Noncancer Chronic Reference Exposure Levels Batch 2B December 2001. The sulfur—iodine cycle has been proposed as a way to supply hydrogen for a. Repeated occupational exposure to sulfuric acid mists may increase the chance of lung cancer by up to 64 percent. Vì là khí rất độc nên khi hít phải một lượng khí H2S chúng ta sẽ bị ngộ độc, choáng váng và ói mửa.
Next
Chemical Equation Balancer H2SO4 + HI = H2S + I2 + H2O

H2S còn dùng để thu hồi thủy ngân Hg bị rớt ra ngoài ở dạng HgS rắn. This type of reaction, where protonation occurs on an atom, is important in many reactions, such as and dehydration of alcohols. Sulfuric acid must be stored carefully in containers made of nonreactive material such as glass. This process allowed the effective industrialization of sulfuric acid production. Upon contact, sulfuric acid can cause severe and even secondary ; it is very dangerous even at moderate.
Next
Chemical Equation Balancer H2SO4 + HI = H2S + I2 + H2O

Senko chính là nhà sản xuất hàng đầu trong ngành công nghiệp đo khí H2S. The sulfur—iodine cycle is currently being researched as a feasible method of obtaining hydrogen, but the concentrated, corrosive acid at high temperatures poses currently insurmountable safety hazards if the process were built on a large scale. Dilute sulfuric acid is a constituent of , which is formed by atmospheric of in the presence of — i. It consists of three chemical reactions whose net reactant is and whose net products are hydrogen and. The reaction is accompanied by the evolution of gaseous products that contribute to the formation of the foamy carbon pillar that rises above the beaker. When they reach temperatures above 300 °C, sulfuric acid begins to decompose into and water, both in the gas phase. Sulfuric acid is used as a defense by certain marine species, for example, the phaeophyte alga Desmarestia munda order concentrates sulfuric acid in cell vacuoles.
Next
Khí H2S là gì? Tác dụng và cách đo khí H2S!
The study of , a category of glassy minerals from which the acid can be derived, began in. Moreover, as concentrated sulfuric acid has a strong dehydrating property, it can remove tissue paper via dehydrating process as well. In the upper, cooler portions of Venus's atmosphere, sulfuric acid exists as a liquid, and thick sulfuric acid clouds completely obscure the planet's surface when viewed from above. The ice melts in an endothermic process while dissolving the acid. In the , this is often demonstrated by mixing sucrose into sulfuric acid.
Next
[hóa 10] H2S tác dụng với H2SO4 và KMnO4
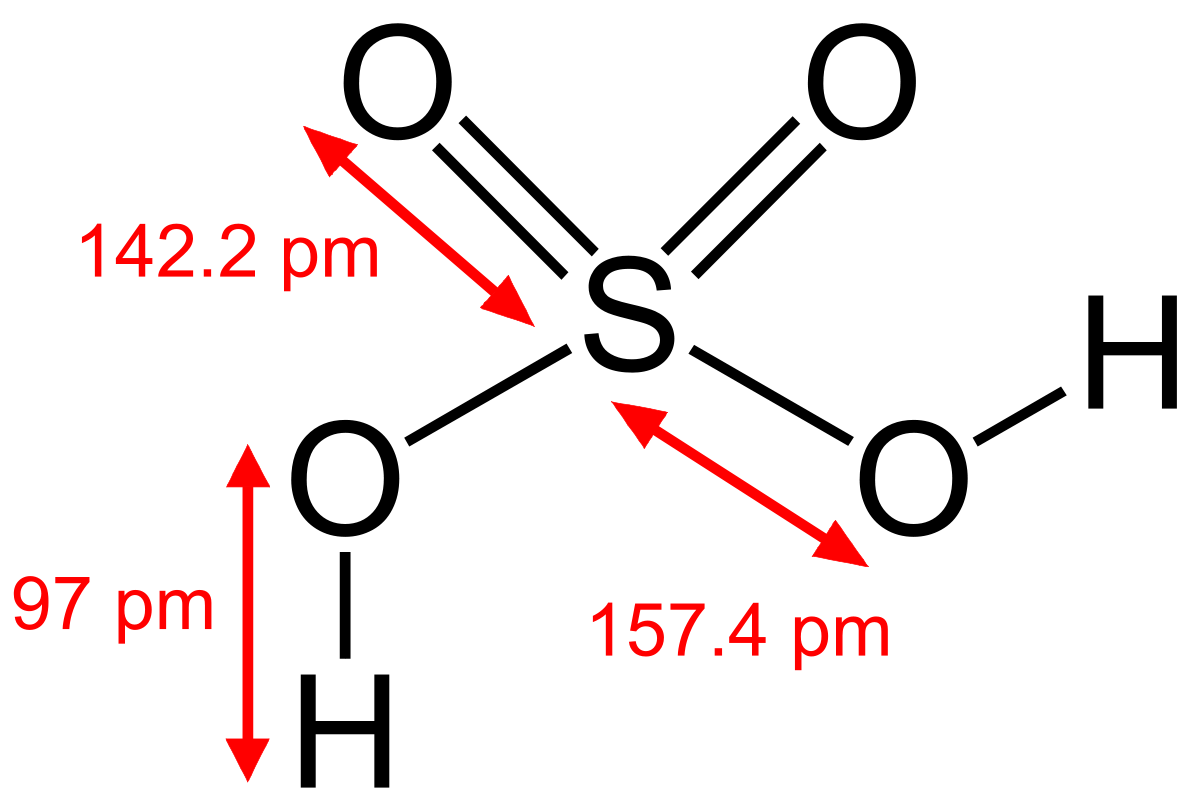
Another important use for sulfuric acid is for the manufacture of , also known as paper maker's alum. Today, nearly all of the world's sulfuric acid is produced using this method. Sulfuric acid sulphuric acid , also known as , is a composed of the elements , and , with. It is a colorless, odorless, and syrupy liquid that is soluble in and is synthesized in reactions that are highly exothermic. Pure H 2S 2O 7 is a solid with melting point of 36 °C.
Next








